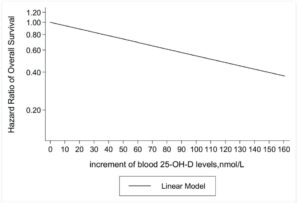
Vitamin D3 deficiency is very common at diagnosis in most cancer types, lung, liver, kidney, triple negative breast cancer, prostate and more. The suggested reasons include cancer related systemic inflammation and even direct dysfunctions in vitamin D3 metabolism linked to the tumor microenvironment. And, often accompanied by low magnesium levels, which affect vitamin D3 metabolism and also need to be corrected and sustained. Both vitamin D3 and magnesium are strongly depleted by inflammation and by many drugs including those used in oncology.
Clinical trials can show mixed results, as with any trials. Nevertheless, there is a clear weight of evidence that higher levels mean lowered risks progression during treatment including in prostate, breast, colorectal, ovarian cancer and others. The VITAL trial is perhaps the most significant so far across many cancer types. At 2000IU daily the results report a 17% reduced progression to metastatic cancer. Importantly, at this dose the effects were 38% in those of low BMI and become insignificant as BMI increases. Researchers suggest higher doses might be considered. In colorectal cancer, there are similar 1/3 or so risk reductions (see References)
Analyses sometimes miss the need for regular and sufficient doses at daily sustained levels to reach significance. And least in breast, colorectal and ovarian cancer treatment, the larger effects are seen at 4000IU and beyond, where meta-analysis across 6000 patients shows significant and increasing relative survival benefits with dose (see Examples). Immunotherapy results for lung, kidney cancer and melanoma, even low supplementation programs have been reported to reduce risk levels very significantly, risk reductions almost a half (see References, PROVIDENCE trial). Very recent research into a variety of immunotherapy drug response rates shows that winter time deficiency in Vitamin D3 has major negative effects, whilst supplementation gave risk reductions 30 percent plus range. (see References) Possibly related, in metastatic clear cell kidney cancer, immunotherapy treatments have very recently been shown to be more effective in the morning.
Vitamin D3 deficiency is associated in several studies with increased metastatic spread. Mainly from research in other diseases, having sufficient levels of vitamin D3 are reported to decrease abnormally high platelet levels, aggregation and adhesion levels with obvious similarity to aspirin or statin actions. All of which are frequently reported mechanisms involved in metastatic spread (click icon for references)
Also taken as Calcitrol, Vitamin D3 is a great opportunity for many. If a dose of 2000, 4000 or 10000IU sounds like a lot, remember that it would be a few minutes of sunshine on a bright summers day. In fact, the aptly named 2019 SUNSHINE trial in advanced colorectal cancer showed doses of 4000IU provided 34% improved survival rates vs 400IU. The “Madden” breast cancer intervention trial confims up to double the relative survival rates for immediate post-diagnosis use. Detailed clinical studies on 10,000 and 40,000IU doses show increasing suppression of prostate cancer (see References). Some clinicial trials are using 10,000IU daily, some 50,000IU weekly. Its important to increase your levels both before and during treatment. 10,000IU or 250 microgram supplements are safe and readily available
Prevention effects of daily vitamin D3 have been reported as 7% risk reduction across the whole population, and 15% in adults aged ≥ 70 years due to a 28% lower risk in men. Probably connected to 12% risk reduction in prostate cancer incidence and 28% in colorectal cancer patients whereas no preventative pre-diagnosis effects were seen in breast and lung cancer patients (see Examples) Similarly in prevention, women in particular see these dose related risk reductions for kidney cancer.