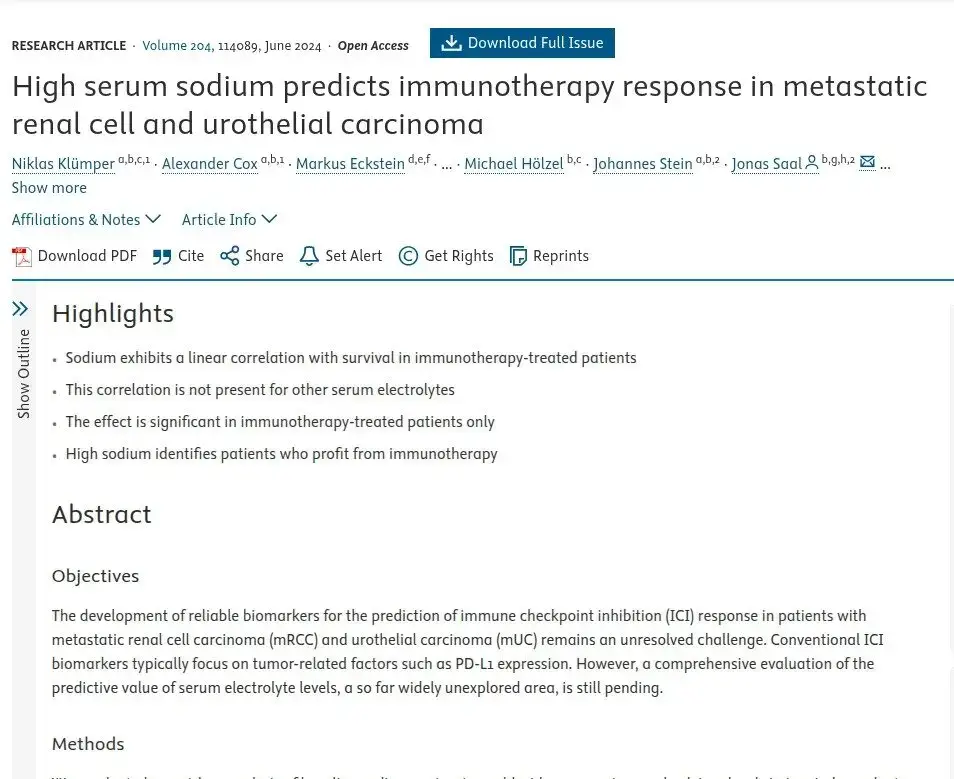
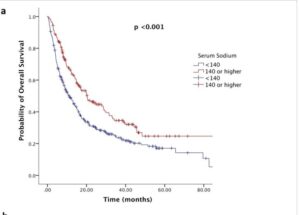
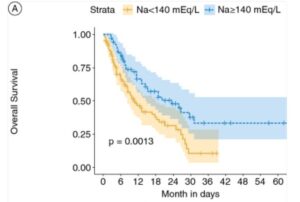
Immunotherapy outcomes, surprisingly, are strongly improved with sufficient and even higher levels of circulating sodium during active therapy windows. In particular studies in metastatic kidney and bladder cancer patient records report some remarkable effects, as well as melanoma and lung cancers. Including for complete theraputic response levels, which are very limited in the low sodium group. Researchers are suggesting sodium level is a more accurate biomarker of success even than PD-L1 immune cell expression levels, the traditional key marker followed in oncology. Furthermore, there are suggestions to actively increase sodium levels before immunotherapy (Highlight 1). But not for other oncology treatment, so far.
There is increasing evidence that having enough sodium in circulation during other oncology treatments helps increase their effects, examples here include recent esophageal cancer patient data for chemo and radiotherapy (Highlight 4) and similar for lung cancer with TKI therapy (References). In advanced metastatic stages studies recommend monitoring and restoring sodum levels based on the observed outcomes vs sodium levels at discharge. Studies highlight the higher natural range of 140 to 145mmol/l. Most treatments and side effects can deplete sodium. Alarmingly, its very common during immunotherapy and there are studies reporting one to two thirds of patients reach deficient levels. Here the data shows more severe lack of sodium after 3 to 5 weeks of therapy in substantial numbers https://pmc.ncbi.nlm.nih.gov/articles/PMC12206762/
This low circulating sodium so called hyponatremia (below 135 mmol/L) is a common finding in cancer patient case data. Its well known as a biomarker for small cell lung cancer. But its fairly common in most cancers and can be worsened by onoclogy treatments, especially chemotherapy. As with many compounds, cancer upregulates its access to sodium to fuel its growth and spread. Inhibiting this activity with re-purposed drugs is an active area of research, including as an anti-metastatic therapy. An example here are emerging pilot studies with lidocaine the surgical anesthetic in head and neck cancers. Research overall suggests that re-balancing circulating sodium levels may help to counter this dysregulated sodium metabolism and aid oncology outcomes.
Beyond immunotherapy, a large meta-analysis in over 3000 patients treated with erlotinib, a tyrosine kinase inhibitor, reported that patients with sufficient sodium , above 135mmol/L , had half the progression risk. And almost identical data has been published for kidney cancer patients treated with TKIs. So very similar to the effects in immunotherapy. A lot of pre-clinical evidence, perhaps surprisingly, shows low sodium to be a marker for increased metastatic spread of cancer too.
Put together, sustaining healthy natural sodium levels is reported to play a crucial role in immunotherapy, and a beneficial effect based on oncology patient data especially in advanced disease. And there are very similar findings in targeted therapy with TKIs. In contrast to this calcium, magnesium and potassium do not affect responses and risk levels. Though of course these minerals can have other important actions to contribute in systemic health.