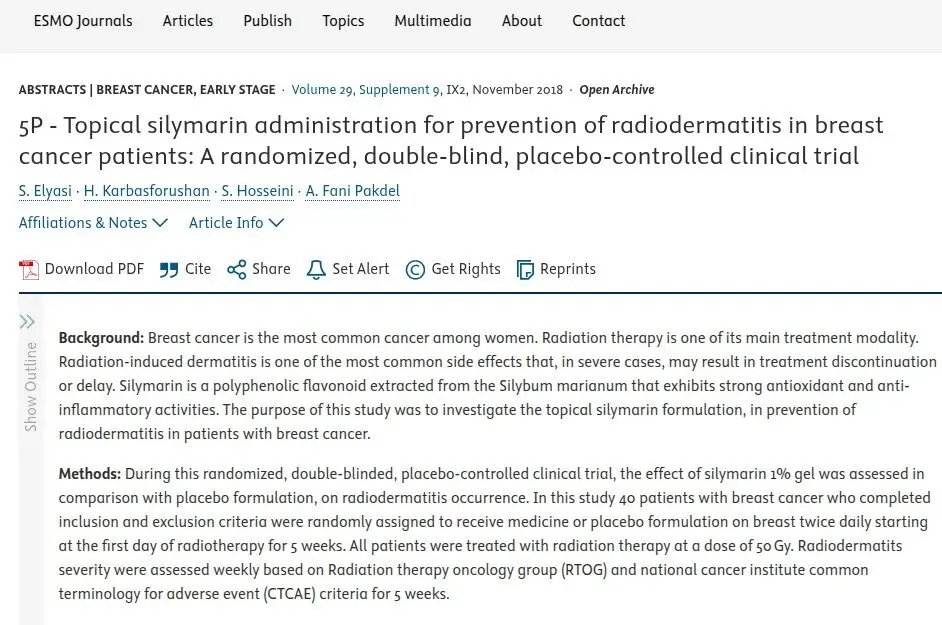
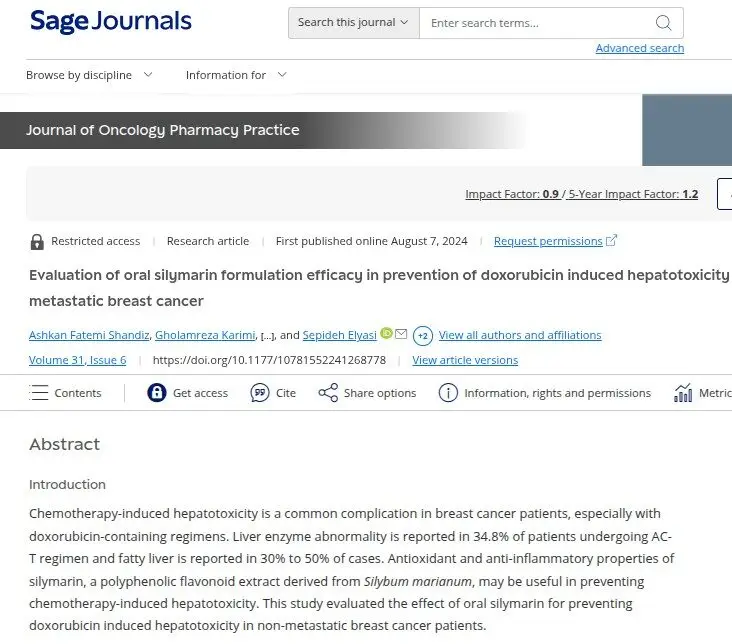
15 – 30%
The form creates your own page where you can describe your plan and upload details of supplements, functional foods and anything else you like to include
UPDATE an existing plan using the form below this one
ALWAYS ANONYMOUS
UPDATES : Select the name of your plan below (“select post”)
Make any changes you want including uploading new files. These will replace your earlier entries.
In case of any issue just reach out using our contact form