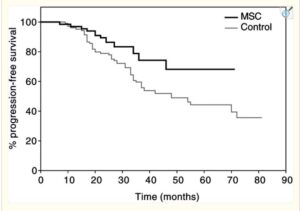
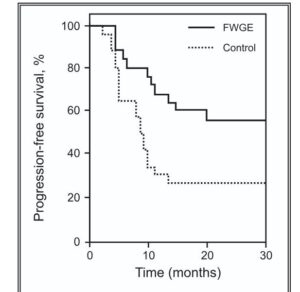
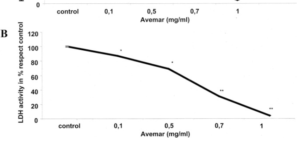
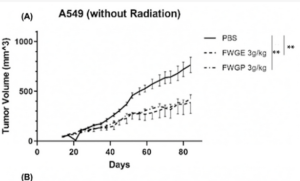
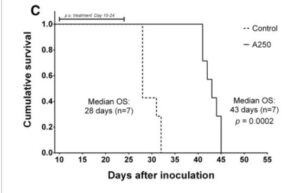
A 2003 clinical trial comparing post-surgical outcomes in colorectal cancer. Avemar branded fermented wheatgerm extracts at 9g per day were used with oncology treatment – chemo/ radation and a larger control group electing not to include Avemar. Notably, the controls – those getting placebo – were very few in advanced stages, while the supplement group were 27% classed as stage IV metastatic CRC. So, the group adding fermented wheatgerm extracts had a much poorer prognosis. Despite this difference, the adjuvant – supporting – therapy with fermented wheatgerm continously over more than 6 months showed surprising overall survival benefits, appearing to be in the 70% range relative improvement over the 5 year plus range. Similar positive effects in terms of progression free disease period, rates of recurrence and formation of new metastases were about a third of the control group.
For comparison , a very large meta-analysis on adding a second line treatment- the largest selling immunotherapy drug bevacizumab https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2734-y The analysis shows an averaged Hazard ratio of 0.86 for overall survival,, indicating roughly 14% relative risk reduction, whilst reporting increased side effects in many cases significant ones.