The 1,3/1,6 form of beta glucan is used in several branded formulae, even prescription products. Derived from mushrooms and increasingly also nutritional yeast sometimes marine algae. Turkey tail mushroom extract has a standalone entry. Beta glucans have a vast amount to research showing how they can help stimulate and balance a healty immune response.
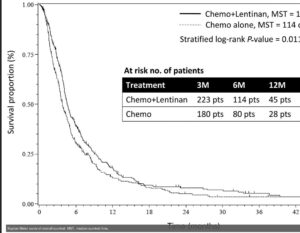
Analysis across several trials reports shiitake mushroom based lentinan with chemotherapy reduces progression risks up to a third in digestive cancers. Results are equivalent for oral supplementation of lentinan as for injectable forms, when both have been tested. Glucans can be taken in substantial amounts too, with good absorption. Digestive tract cancers such as colorectal and gastric have the most research, but there are additional studies in lung cancer, melanoma, even recently with CD40 inhibitors for pancreatic cancer. Often the effects are relatively stronger during more advanced stages.
Many of the most damaging oncology drug side effects can be reduced, frequently halved. This is due to the protective actions of glucans on immune system cells, seen in markers like white blood cell count. Reports vary from a few percent to a 50% effect in patients, of course depending on the oncology drugs and regime needed. Branded pharma grade products such as odetiglucan (Imprime PGG) are reported to improve outcomes in immunotherapy. Both directly, by enhancing the immune system response the tumor itself and reducing resistance, and also indirectly by limiting damaging side effects. And, almost halving the progression of triple negative breast cancers treated with pembrolizumab (Keytruda), look into the Examples below. Trials are ongoing for improving lung, colorectal, Non-Hodgkin lymphoma and breast cancer therapy already. Nichi-glucan are also yeast derived refined glucan, available from Japan https://gncorporation.com/en/shop-top-en/ , with strong evidence for improved immune system health.
High grade commercially available products derived from yeast including Saccharomyces cerevisiae include those based on Wellmune such sa California Gold Immune Defense, or Yestimun, such as Biogens. And brands such as Immiflex and others. There are also branded refined mushroom based products such AHCC from shiitake, which has recent positive trials in clearing HPV infections that drive cervical cancer recurrence. Also improving outcomes in liver cancer including better quality of life during chemotherapy (see References). There is abundant evidence in clinical studies alongside oncology drugs for improving blood markers, immune health and quality of life. Start a regular dose to help limit immune inflammatory response before oncology therapies and consider higher doses if needed once treatments start.
Glucans from processed oats are distinctly different, and are FDA approved for their cholesterol reduction. Excess cholesterol is linked to progression. These help improve microbiome health which can be a crucial factor in outcomes (see Pathfinder examples)