A 2016 national scale Danish study followed well over a hundred thousand patients between 1995 and 2011 following a diagnosis of metastasized non-localized cancers, with particular focus on advanced lung cancer. Here, the researcher were evaluating so called CAD class of compounds, able to increase direct cell death in tumor cells. And, looking for the ability to increase the effectiveness of first line chemo therapy by reducing resistance mechanisms to oncology drugs using antihistamines.
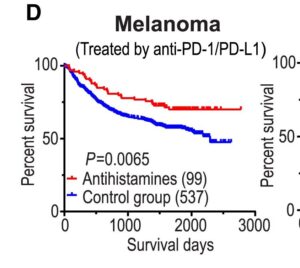
Approximately 2.5% of patients were users of non-CAD class anti-histamines and a smaller 1.5% used mainly ebastine, loratadine, desloratadine and the now withdrawn astemizole For non localized advanced cancers, the detailed report does shows ONLY loratadine with clear tendency to reduce relative mortality even without chemotherapy. What is more impressive, are the results when used in patients that also underwent chemotherapy. Here all three of ebastine, desloratadine and loratadine all had significant effects, reducing relative risk of mortality over 20%
The study focuses more deeply into metastatic lung cancer, where only loratadine and ebastine were able to increase relative survival rates. Striking results were shown, with a 30 to 40% increase in relative survival rates for this group beyond those of chemotherapy alone. So a very large effect, similar or better than adding immunotherapy drugs to chemo, but with no side effects. This is a strong validation of the direct cancer killing potential of these carfefully screened antihistamines.