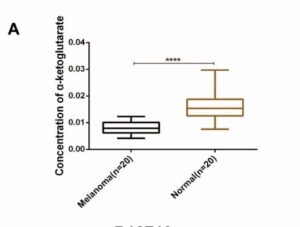
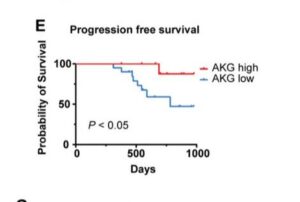
Analysis of patient data from large scale immunotherapy trials including melanoma and clear cell kidney cancer shows higher patient levels of alpha ketoglutarate (AKG) lead to much better treatment responses. AKG is a naturally circulating kind of keto-acid, and commonly available supplement sometimes called 2-oxoglutarate, popular in anti-aging science. Its produced by enzymes breaking down foods including dairy. In particular, AKG can help improve or rejuvenate mitochondrial health, and target uses include long term kidney, muscle, skin and tissue health. In cancer, AKG is emerging in lab trials as compound increasing responses during immunotherapy in particular, but potentially radio and chemotherapies too
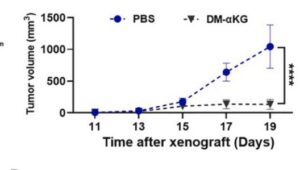
Recent pre-clinical study reports higher immunotherapy response are found in melanoma patients with higher αKG levels. Compelling in-vivo testing confims that boosting levels during PD-1 therapy significantly increase response rates and disease free survival – nivolumab, pembrolizumab and more. The AKG enhanced immune response via interferon gamma INFγ-STAT1/3 signaling is explained, a process also seen in lung cancer progression. Very similar finding have been made in lymphoma and clear cell kidney cancer (see References). Pre-clinical work uses DMKG, an αKG derivative used in research studies which can penetrate cells directly. For instance, amplifying radiotherapy outcomes, reducing TNBC treatment evasion utilizing the burst in autophagy bought on by radation.
Both with immunotherapy and radiotherapy, lab results show increasing levels break down tumor resistance to treatment. AKG is metabolized quickly and effects are sustained at least a day. Some of the studies show tumor growth suppression by AKG alone, and suggest likely improvement in chemotherapy. And other pre-clinical studies strongly suggest anti-metastatic activity, which is yet to be proven in clinical trials. Whats important here is to ramp up and sustain alpha ketoglutarate before and during immunotherapy.
There are fascinating ongoing clinicial trials into the health and lifespan extending actions of AKG, based on its ability to re-regulate mitochondrial health. Research also shows that when glucose restriction drugs are used to suppress tumor growth, low levels of AKG result. And this drives mutation to more aggressive and resistant behaviors which have been observed when glucose inhibitors are removed during trials on pancreatic cancer. Future research may expand on how to optimize the use of several drugs and compounds.