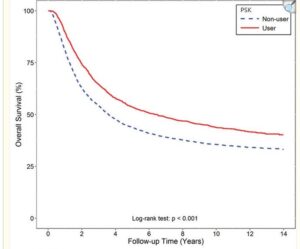
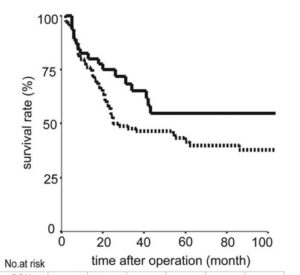
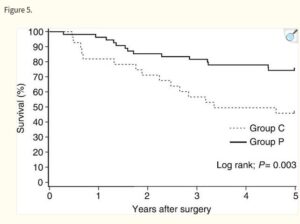
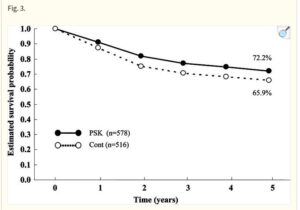
This 2022 study uses patient data from the Tiawan national health insurance database to compare survival rates of turkey tail + chemotherapy regimes with chemotherapy alone. Nearly 6500 or gastric cancer patients from 1999 to 2008 were compared, of which 1300 had used PSK from Turkey Tail at average 3g daily as an adjuvant to oncology. Post operative survival time was of 6.5 years with turkey tail plus chemo. Close to 2X the 3.6 years average survival of larger group of patients with various standard oncology treatments alone. A relative risk reduction of 24% was calculated. The authors comment “Prospective studies regarding the tumor microenvironment and clinical trials of PSK in combination with modern immunotherapeutic agents are warranted.”
As a comparison, the adding the most common immunotherapy drug nivolumab in gastric cancer to chemotherapy has lower 21% reduction in relative risk across all patients. . . https://www.nature.com/articles/s41586-022-04508-4
Its feasible, or even likely, that PSK/ Turkey tail use as an adjuvant therapy in late stage gastric cancers may, for some at least, have similar outcomes in survival to even recently introduced drugs, like angiogenisys inhibitors. Of course without the significant side effects risks. And, for some, increase the possibilities of longer terms of disease free progression alongside immunotherapies.