Most studied is colorectal cancer often reporting relative risk reductions in progression rates. Some higher responders are identified in recently published Nordic multi-site ALASCCA clinical trials. Here, the 37% of patients with alterations to so called PI3K pathway signaling saw the substantial benefits (see References). Dysregulated PI3K is common, and similar levels are seen in breast, cervical, gastric and other cancers. Meta-analysis of patient records across breast, prostate, lung cancers see tendency to positive effects in the 9-12%. Kidney cancer patients also see 9%, (19% when supporting mTOR inhibitors) In lower numbers of case controlled analyses, promising responses are reported for ovarian and bladder cancers, and quite striking improved survival in gliomas, melanoma, head and neck cancers – even leukemia.
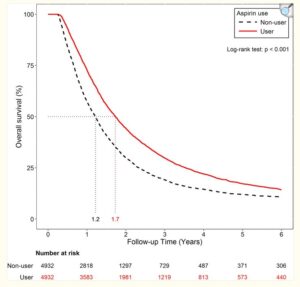
Over 30 clinical trials are ongoing, alongside chemotherapy, tyrosine kinase inhibitors and immunotherapies. Mainly in colorectal and gastric cancer, with some in prostate, breast and ovarian cancer. Only statins have more investment and focus with >50 trials. Analysis of overall patient drug regimes during immunotherapy treatments, such as pembrolizumab, show that those taking aspirin – but not other anti-coagulants – had significantly higher response rates and lower risk levels. Statins, in particular, were the other main category showing benefits , while drugs others such as metformin or diprydamole do not. Aspirin can also improve cholesterol reducing effects of statins. Other studies point to side effects increasing with aspirin use during immunotherapy, important to be aware of.
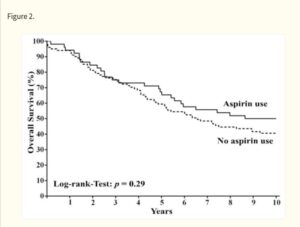
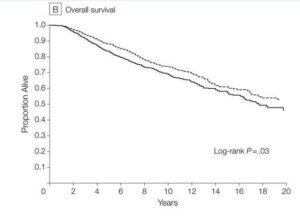
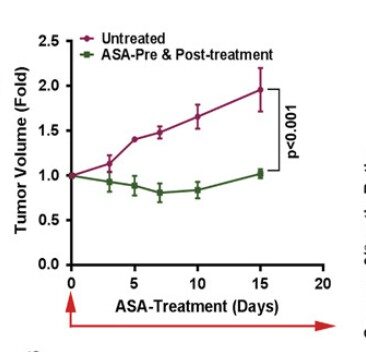
Aspirin has well proven anti-metastatic activity including suppression of the “clotting” enzyme thromboxane brings down excess levels of platelets seen in metastasis of digestive tract, breast, lung and other cancers. Especially important in post-surgery setting and subject of a sub-study in ongoing ADD-ASPIRIN clinical trial. Some large scale studies show a remarkable association of aspirin to reduce cancer spread, leading to improved progression free survival for instance(Highlight4). There are studies showing synergies with statins. (Click the icon below for further references)
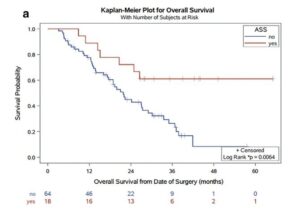
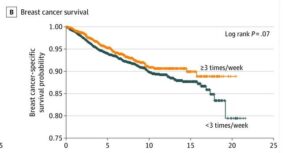
Pre-existing inflammatory conditions indicate likely response and more clear benefit from post-diagnostic use. An example here can be found in prostate cancer trials where improved outcomes are found in patients taking low dose aspirin for cardiovascular risk. Also a recent publications in Nature Journal summarizing the overall science emphatically. (see References)
In prevention a 2024 UK meta-analysis sees nine cancer types with significantly lower incidence for those diagnosed with one or more systemic inflammatory diseases. Meaning metabolic (diabetes etc), autoimmune (rheumatic, digestive, skin), cardiovascular, neuro-degenerative and more. Those combining aspirin and statin use in that group, saw several more risk reduction effects including breast, liver kidney and ovarian cancer. Aspirin is a well known inhibitor of so called COX enzymes which feature heavily in both inflammatory disease and progress of cancers.
For otherwise healthy individuals, there was no overall risk reduction is found for low dose aspirin. However, medium to higher dose users over 100mg daily saw slightly increased risk in a few cancers, notably multiple myeloma. Regular or full dose aspirin use can raise risk, for instance prostate cancer. Large population studies also comment on the risk of bleeding, especially in older patient groups. For low doses, the effects are small but as always – always seek qualified medical advice. At least one analysis shows increased risks in some cancer incidence in older patient groups (see concerns)